Abstract
Background: Ibrutinib (IBR), a BTK inhibitor is approved for patients (pts) with CLL. Venetoclax (VEN), a BCL2 inhibitor is approved for pts with CLL who have received prior therapy and have del(17p). The rationale for combining IBR and VEN includes: 1) preclinical models showing synergism with combined IBR and VEN (Cervantes-Gomez, CCR. 2015), 2) non-overlapping toxicity profiles; 3) non-overlapping mechanisms of action; 4) complementary activity in treating disease compartment. We report preliminary results of an investigator-initiated phase II trial of combined IBR and VEN for pts with CLL (NCT02756897).
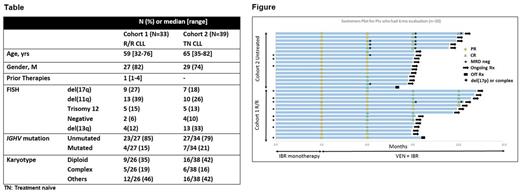
Methods: Two pt cohorts were enrolled; Cohort 1 (relapsed/refractory CLL); Cohort 2 (untreated pts with at least one high-risk feature: del(17p), mutated TP53, del(11q), unmutated IGHV, ≥65 yrs). Eligibility criteria included age ≥18 yrs, ECOG PS ≤2, adequate renal (creatinine clearance >50 mL/min) and hepatic function (ALT and AST ≤3.0 x upper limit of normal (ULN), total bilirubin ≤1.5 x ULN). Treatment consisted of IBR monotherapy 420mg daily for the first 3 months, followed by addition of VEN (weekly dose escalation to the target dose of 400mg daily). IBR may be continued indefinitely; VEN for a total of 2 yrs. The primary endpoint is achievement of CR/CRi. A total of 78 pts are planned (39 in each Cohort). Response assessment is per IWCLL 2008 criteria with bone marrow and CT scans every 3 months during the first year, then every 6 months. Minimal residual disease (MRD) was assessed by 4-color flow cytometry in bone marrow (sensitivity 10-4).
Results: Since August 2016, 72 pts initiated treatment (Cohort 1, n=33; Cohort 2, n=39). Median follow-up is 7.5 months (range, 0.6-12.1). The baseline characteristics are shown in the Table. Overall, 61 pts have completed IBR monotherapy, and have initiated VEN dose escalation. Three months of IBR monotherapy resulted in 51% (31/61) down-grade of TLS risk category; there were 2/61 (3%) high-risk for TLS at the time of initiation of VEN.
In Cohort 1, 29 pts received IBR monotherapy then initiated VEN dose escalation. All 14 pts who completed at least 3 months of combination therapy had a response (9 CR/CRi, 5 PR). There was a significant decrease in bone marrow infiltrate with the addition of VEN (Figure), including MRD-negativity or MRD <0.1% in several pts with high-risk cytogenetics.
In Cohort 2, 32 pts received IBR monotherapy then initiated VEN dose escalation. All 16 pts who completed at least 3 months of the combination therapy had a response (9 CR/CRi, 7 PR). Several of these pts achieved undetectable bone marrow MRD status with addition of VEN (Figure).
Overall, 24% pts required dose reduction of IBR, 18% required dose reduction of VEN. The most common reason for dose reduction was neutropenia. Eight pts (11%) developed atrial fibrillation, likely secondary to IBR. One pt had laboratory TLS. No pt had clinical TLS. A total of 10 pts have come off study (Cohort 1, n=5; Cohort 2, n=5); 6 during the IBR monotherapy phase due to skin rash (n=2), hypertension and gait imbalance (n=1), need for chronic azole therapy (n=1), infection (n=1), and consent withdrawal (n=1); 4 after starting VEN due to cytopenia (n=1), Hodgkin's transformation (n=1), hypertension (n=1), and second malignancy (fallopian tube cancer) (n=1).
Conclusions: The combination of IBR and VEN is safe and active in pts with CLL. These early results of efficacy are highly encouraging. Significant improvement in bone marrow CLL infiltrate is noted with several pts achieving undetectable MRD status as early as 3 months of the combination therapy.
Jain: ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Verastem: Research Funding; BMS: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Incyte: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees. Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Burger: Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Takahashi: Symbio Pharmaceuticals: Consultancy. Bose: Incyte Corporation: Honoraria. Jabbour: Bristol-Myers Squibb: Consultancy. DiNardo: Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding. Cortes: ImmunoGen: Consultancy, Research Funding; Sun Pharma: Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Kantarjian: Amgen: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding. Wierda: Karyopharm: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Kite: Research Funding; Janssen: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Juno: Research Funding; The University of Texas MD Anderson Cancer Center: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal